Cholinergic blocking drug
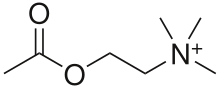
Cholinergic blocking drugs are a group of drugs that block the action of acetylcholine (ACh), a neurotransmitter, in synapses of the cholinergic nervous system.[1] They block acetylcholine from binding to cholinergic receptors, namely the nicotinic and muscarinic receptors.
These agents have broad effects due to their actions in nerves located vastly over the body. These nerves include motor nerves in somatic nervous system which innervate skeletal muscles as well as nerves in the sympathetic and parasympathetic nervous systems.[1] Organs that receive innervations from these systems include exocrine glands, heart, eyes, gastrointestinal tract etc. Antimuscarinic and antinicotinic agents can increase heart rate, inhibit secretions, and gastrointestinal motility.[1][2]
Naturally occurring antimuscarinics were found in alkaloids from Belladonna (Solanaceae) plants. They were used as deadly poison and pupil-dilating cosmetics. While curare, the naturally occurring antinicotinics derived from Chondrodendron and Strychnos, was a poison used by South American Indians for hunting.[1][3][4]
According to their site of actions, cholinergic blocking drugs can be classified into two general types — antimuscarinic and antinicotinic agents.[1] Antimuscarinic agents (also known as muscarinic antagonists), including atropine and hyoscine, block acetylcholine at the muscarinic acetylcholine receptors. Antinicotinic agents (also known as ganglionic blockers, neuromuscular blockers), including tubocurarine and hexamethonium, block acetylcholine action at nicotinic acetylcholine receptors. Their effects are based on the expression of corresponding receptors in different parts of the body.
There are many adverse effects, interactions and contraindications for antinicotinic and antimuscarinic agents. Adverse effects include hypotension, dry mouth, dry eyes etc. They interact with grapefruit juice and various medications, e.g. warfarin, metoclopramide. Therefore, cautions should be exercised and advice from medical professionals should be sought before using medications.
History
[edit]Discovery of cholinergic nervous system
[edit]In 1900, Reid Hunt, a pharmacologist (1870-1948), realised a fall in blood pressure in rabbits after removing adrenaline (epinephrine) from adrenal glands extract. While he initially attributed this effect to choline, he later discovered acetylcholine was 100 000 times more potent in lowering blood pressure.[2]

British physiologist Sir Henry Hallett Dale (1875-1968) observed acetylcholine for causing blood vessel dilation and slowing down heart rate. In 1914, Dale noted that the physiological effect of acetylcholine resembled the stimulation of parasympathetic nervous system and hypothesized acetylcholine as the neurotransmitter. Later, Dale named substances that mimic acetylcholine action as "cholinergics".[5]
In 1914, Dale also distinguished two types of activities of acetylcholine, namely muscarinic and nicotinic, as they mimic the effects of injecting muscarine, extracted from poisonous mushroom Amanita muscaria, and nicotine.[2]
Antimuscarinic agents
[edit]Naturally occurring antimuscarinics were found in alkaloids from Belladonna (Solanaceae) plants. They were used as deadly poison in Roman Empire and Middle Ages. The name Belladonna, meaning beautiful ladies, was derived from women using berry juice from the plant cosmetically to dilate their pupils.[4]
The mydriatic effect was studied by the German chemist Friedlieb Ferdinand Runge (1795-1867), in which the active ingredient, atropine, was first discovered by Vaquelin in 1809 and was first isolated by Heinrich F. G. Mein in 1813.[4]
In the 1850s, atropine was used as antispasmodic in asthma treatment and as morphine antidote for its mydriatic effect.[4] Bezold and Bloebaum showed that atropine blocked the effects of vagal stimulation on the heart in 1867. Subsequently in 1872, Heidenhain found its ability to prevent salivary secretion.[6]
Antinicotinic agents
[edit]Curare, derived from Chondrodendron and Strychnos, was used as poison by South American Indians to coat arrow tips or blow-pipe darts for hunting animals. It is first identified when Spanish soldiers were attacked by these indigenous tribes in the 16th century.[1][3]
In 1906, Langley studied the actions of nicotine and curare on chicken and frog muscles. Curare was found to block the stimulant action of nicotine in both innervated and chronically denervated muscles. In 1940, Jenkinson identified tubocurarine as a competitive antagonist of acetylcholine.
Curare and tubocurarine had important roles in establishing the concept of specific cholinoceptors in the motor end plate.[3] At right dose, they are used as general anesthetic for relaxing abdominal muscles in operations.[1]
General effects on body
[edit]Antimuscarinic agents
[edit]Muscarinic receptors are G-protein coupled receptors that present mainly in the parasympathetic system and sweat gland. Antimuscarinc agents, therefore, generally produce effects that are opposite to the stimulation of the parasympathetic system, which is responsible for "rest and digest".[1][2]
| Location | Effects |
|---|---|
| Exocrine glands |
|
| Cardiovascular system |
|
| Eye |
|
| Gastrointestinal tract |
|
| Other smooth muscle |
|
| Central Nervous System (CNS) |
|
Antinicotinic agent
[edit]Nicotinic receptors are ligand-gated ion channels that present in both parasympathetic and sympathetic ganglions, while the antagonistic effect of antinicotinic agents depend on which system predominates in a particular site. Nicotinic receptors are also present in neuromuscular junctions and the brain.[1][2]
| Location | Predominant system | Effects |
|---|---|---|
| Exocrine glands | Parasympathetic except sweat glands |
|
| Heart | Parasympathetic |
|
| Blood vessels | Sympathetic |
|
| Eye | Parasympathetic |
|
| Gastrointestinal tract | Parasympathetic |
|
| Other smooth muscle | Parasympathetic |
|
| Neuromuscular junction | N/A |
|
Clinical uses
[edit]Listed below are some examples of antimuscarinic and antinicotinic agents according to the British National Formulary, including non-clinically one for better illustration of their site of actions.[7]
Antimuscarinic agents
[edit]Antimuscarinic agents are muscarinic antagonists and they bind to muscarinic cholinergic receptors postsynaptically without activating them. They occupy and prevent acetylcholine from binding to the active sites of receptors to elicit their effect.[1][2]
| Examples | Properties | Clinical use | Notes |
|---|---|---|---|
| Atropine |
|
Ophthalmologic examination
Surgery Premedication[8]
Myopia
Acute symptomatic bradycardia
|
|
| Glycopyrrolate |
|
Hyperhidrosis
|
|
| Dicycloverine (Dicyclomine) |
|
Bowel Colic
|
|
| Hyoscine (Scopolamine) |
|
Motion sickness
Bowel Colic
|
|
| Tiotropium |
|
Asthma and Chronic Obstructive Pulmonary Disease (COPD)
|
|
| Ipratropium | |||
| Tropicamide |
|
Ophthalmologic examination
|
|
| Cyclopentolate | |||
| Darifenacin |
|
Urinary incontinence
|
|
Antinicotinic agents
[edit]Antinicotinic agents are classified into ganglionic blockers and neuromuscular blockers.
Ganglionic blockers are of little clinical use as they act at all autonomic ganglions.[1][2] They act by:
- Interfering acetylcholine release
- Prolonged depolarization (depolarisation block), i.e. stimulation then block stimulation
- Competitive inhibition of nicotinic receptor
| Examples | Mechanism of action | Properties | Clinical use |
|---|---|---|---|
| Nicotine | Prolonged depolarization |
|
Smoking Cessation
|
| Acetylcholine (in presence of cholinesterase inhibitors) | No clinical use as ganglionic blocker | ||
| Hexamethonium | Competitive inhibition of nicotinic receptor |
|
No longer clinically use due to side effect |
| Trimethaphan |
|
Blood pressure lowering in surgery (rarely use) | |
| Tubocurarine |
|
Rarely used | |
| Atracurium |
|
Surgical anaesthetic & intubation
|
Neuromuscular blockers act at neuromuscular junction by:[1][2]
- Inhibiting acetylcholine synthesis
- Inhibiting acetylcholine release
- Blocking acetylcholine receptors postsynaptically
- Prolonged depolarization of motor end plate
| Examples | Mechanism of action | Onset | Duration of action | Properties | Clinical use |
|---|---|---|---|---|---|
| Hemicholinium | Inhibiting acetylcholine synthesis | / | / |
|
No clinical use |
| Vesamicol |
|
No clinical use | |||
| Botulinum toxin | Inhibiting acetylcholine release | 3–5 days | 3–4 months |
|
Muscle relaxants
Reduce secretion
Headache prophylaxis
|
| Beta-bungarotoxin | / | / |
|
No clinical use | |
| Tubocurarine | Blocking acetylcholine receptors postsynaptically | Slow
(> 5mins) |
Long (1-2h) |
|
Rarely used |
| Alcuronium |
|
No clinical use currently[15] | |||
| Pancuronium | Intermediate (2-3 min) | Long (1-2h) |
|
Surgery Premedication
Euthanasic agent
| |
| Pipecuronium |
|
Surgery Premedication
| |||
| Vecuronium | Intermediate | Intermediate
(30-40min) |
|
Surgery Premedication
| |
| Rocuronium |
| ||||
| Atracurium | Intermediate | Intermediate
(<30 min) |
| ||
| Doxacurium |
| ||||
| Cisatracurium |
| ||||
| Mivacurium | Fast (~2mins) | Short (~15 mins) |
| ||
| Suxamethonium | Prolonged depolarization of motor end plate | Fast | Short |
| |
| Rocuronium |
|
Adverse effects
[edit]Drug reactions
[edit]The following are some side effects after taking either antinicotinic or anticholinergic medications. They vary from mild to severe and some of these effects depends on the duration of drug usage.
Cognitive function decline (Confusion, memory loss and difficulty in concentration)[18] paralysis, Tachycardia,[19] Hypotension (Anticholinergics are histamine-inducing, leading to vasodilation during anaphylactic reaction, hence a dropping in blood pressure),[20] constipation, dry mouth, dry eyes, hypohidrosis/ anhidrosis, blurry vision, or Increase in intraocular pressure, increase in the risk of glaucoma.
Overdose
[edit]Anticholinergic overdose, both antinicotinic and antimuscarinic, can exert toxic effects on both central and peripheral systems. The following symptoms could be presented:[21][22]

Mild symptoms include tachycardia, flushed face, mydriasis and blurred vision, fever, dry mouth and skin, and urinary retention. Early stage of overdose can lead to central nervous system stimulation, for instance, hyperactivity, followed by depression, such as agitation (Anxiety or nervous), delirium, disorientation, hallucinations, seizures, hypertension, or hyperthermia. In late or severe stage of overdose, it could lead to coma, medullary paralysis, death.

Supportive care is usually performed in anticholinergic toxicated patients. Intravenous benzodiazepine is used as a first-line treatment for agitation. Cooling measures are employed if there is any significant hyperthermia. Activated charcoal is only given within one hour of anticholinergic ingestion. Physostigmine is given only if presenting both peripheral and central signs and symptoms of anticholinergic poisoning.[23] Physostigmine is a central and peripheral acting acetylcholinesterase inhibitor and generally given to patients with pure anticholinergic poisoning.[24]
Interactions
[edit]Combined use of medications with anticholinergics may cause synergistic (supra-additive), additive, or antagonistic interactions, leading to no therapeutic effect or overdosing.[25][26] Below listed are some medications or food that can interact with anticholinergics.
Medications indicated for:
- Irregular heartbeat, e.g. disopyramide, quinidine. Drug-induced arrhythmia worsened by anticholinergics' side effect of tachycardia.
- Parkinson's disease, e.g. levodopa. Atropine decreases the absorption of levodopa.
- Preventing travel sickness, relieve stomach cramps or spasms, e.g. hyoscine. Additive effect.
- Nausea and vomiting, e.g. cyclizine. Additive effect.
- Parasympathetic stimulation, e.g. bethanechol, pilocarpine, carbachol
- Antihistamines e.g. chlorpheniramine, diphenhydramine, promethazine. They have similar structures as anticholinergics, causing additive effect.
- Tricyclic antidepressants, e.g. amitriptyline, clomipramine. Additive effect.
- Adrenergic decongestants, e.g. phenylephrine. Combined use with atropine increases the risk of severe hypertension.
- Alzheimer's disease e.g. rivastigmine and donepezil. May reduce seizure threshold.[27]
- Muscle relaxants for surgery.
- Grapefruit juice and grapefruit-based products. CYP3A4 inhibitor which may reduce or amplify drug effect, such as darifenacin.
Contraindications
[edit]The followings are the common contraindications adopted from the British National Formulary.[7]
Antimuscarinic agents
[edit]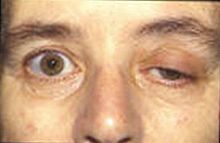
For all antimuscarinics,
- Angle-closure glaucoma
- Bladder outlet obstruction (BOO)
- Myasthenia gravis
- Gastro-intestinal obstruction
- Toxic megacolon
- Urinary retention
- Paralytic ileus
- Intestinal atony (paralysis of muscles)
- Severe ulcerative colitis
- Hypertension, especially M2 receptor antagonists
Antinicotinic agents
[edit]For anticholinergics, such as
- Trimethoprim:
- Suxamethonium:
- Hyperkalemia
- Low plasma-cholinesterase activity e.g. severe liver disease
- Major trauma
- Personal or family history of congenital myotonic disease
- Personal or family history of malignant hyperthermia
- Prolonged immobilisation
- Severe burns
- Skeletal muscle myopathies e.g. Duchenne muscular dystrophy
References
[edit]- ^ a b c d e f g h i j k l Patrick G (2019-10-10). "Introduction". Introduction, Medicinal Chemistry. Taylor & Francis. pp. 2–3. doi:10.1201/9780429188572-1. ISBN 978-0-429-18857-2. S2CID 243582955.
{{cite book}}:|work=ignored (help) - ^ a b c d e f g h "Preface". Rang and Dale's Pharmacology (7Th ed.). Elsevier. 2012. pp. xv. doi:10.1016/b978-0-7020-3471-8.00064-0. ISBN 978-0-7020-3471-8.
- ^ a b c Bowman WC (January 2006). "Neuromuscular block". British Journal of Pharmacology. 147 Suppl 1 (S1): S277-86. doi:10.1038/sj.bjp.0706404. PMC 1760749. PMID 16402115.
- ^ a b c d Shutt LE, Bowes JB (May 1979). "Atropine and hyoscine". Anaesthesia. 34 (5): 476–90. doi:10.1111/j.1365-2044.1979.tb06327.x. PMID 382907. S2CID 41496486.
- ^ Raju TN (2014). "Dale, Henry Hallett". Encyclopedia of the Neurological Sciences. Elsevier. pp. 926–927. doi:10.1016/b978-0-12-385157-4.00848-4. ISBN 978-0-12-385158-1.
- ^ Behcet A (2014-03-25). "The Source-Synthesis- History and Use of Atropine 1". Journal of Academic Emergency Medicine. 13 (1): 2–3. doi:10.5152/jaem.2014.1120141. ISSN 1305-760X.
- ^ a b "Digital Medicines Information Suite". MedicinesComplete. Retrieved 2021-03-05.
- ^ a b Gallanosa A, Stevens JB, Quick J (2021). "Glycopyrrolate". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30252291. Retrieved 2021-03-05.
- ^ Upadhyay A, Beuerman RW (May 2020). "Biological Mechanisms of Atropine Control of Myopia". Eye & Contact Lens. 46 (3): 129–135. doi:10.1097/ICL.0000000000000677. PMC 7176345. PMID 31899695.
- ^ "Part 7.3: Management of Symptomatic Bradycardia and Tachycardia". Circulation. 112 (24_suppl): IV–67-IV-77. 2005-11-28. doi:10.1161/circulationaha.105.166558. ISSN 0009-7322.
- ^ "Assessment of Safety, Tolerability and Efficacy of 1% GPB Cream Versus Qbrexza® (Glycopyrronium) Cloth 2.4% Under Maximum-Use Conditions in Subjects With Primary Axillary Hyperhidrosis". Case Medical Research. 2019-11-11. doi:10.31525/ct1-nct04159610. ISSN 2643-4652. S2CID 243333125.
- ^ Stein JM (September 1975). "The effect of adrenaline and of alpha- and beta-adrenergic blocking agents on ATP concentration and on incorporation of 32Pi into ATP in rat fat cells". Biochemical Pharmacology. 24 (18): 1659–62. doi:10.1016/0006-2952(75)90002-7. PMID 12.
- ^ "Hemicholinium-3". PubChem. Retrieved 2021-03-05.
- ^ Escher CM, Paracka L, Dressler D, Kollewe K (February 2017). "Botulinum toxin in the management of chronic migraine: clinical evidence and experience". Therapeutic Advances in Neurological Disorders. 10 (2): 127–135. doi:10.1177/1756285616677005. PMC 5367647. PMID 28382110.
- ^ Hillier K (2007). "Alcuronium". X Pharm: The Comprehensive Pharmacology Reference. Elsevier. pp. 1–4. doi:10.1016/b978-008055232-3.61181-x. ISBN 978-0-08-055232-3.
{{cite book}}:|work=ignored (help) - ^ Riley S (August 2017). "Navigating the new era of assisted suicide and execution drugs". Journal of Law and the Biosciences. 4 (2): 424–434. doi:10.1093/jlb/lsx028. ISSN 2053-9711.
- ^ Preuss C (2010). "Pipecuronium". xPharm: The Comprehensive Pharmacology Reference. Elsevier. pp. 1–5. doi:10.1016/b978-008055232-3.63925-x. ISBN 978-0-08-055232-3.
- ^ Fox C, Smith T, Maidment I, Chan WY, Bua N, Myint PK, et al. (September 2014). "Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review". Age and Ageing. 43 (5): 604–15. doi:10.1093/ageing/afu096. PMID 25038833.
- ^ Moss J (August 1995). "Muscle relaxants and histamine release". Acta Anaesthesiologica Scandinavica. Supplementum. 106: 7–12. doi:10.1111/j.1399-6576.1995.tb04301.x. PMID 8533551. S2CID 37305853.
- ^ Hunter JM (July 1993). "Histamine release and neuromuscular blocking drugs". Anaesthesia. 48 (7): 561–3. doi:10.1111/j.1365-2044.1993.tb07114.x. PMID 7688493. S2CID 36119841.
- ^ Broderick ED, Metheny H, Crosby B (2021). "Anticholinergic Toxicity". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30521219.
- ^ National Research Council (US) Panel on Anticholinesterase Chemicals; National Research Council (US) Panel on Anticholinergic Chemicals (1982-01-01). Possible Long-Term Health Effects of Short-Term Exposure to Chemical Agents. doi:10.17226/740. ISBN 978-0-309-07759-0. PMID 25032448.
- ^ Derinoz O, Emeksiz HC (September 2012). "Use of physostigmine for cyclopentolate overdose in an infant". Pediatrics. 130 (3): e703-5. doi:10.1542/peds.2011-3038. PMID 22908101. S2CID 22609464.
- ^ Pentel P, Peterson CD (November 1980). "Asystole complicating physostigmine treatment of tricyclic antidepressant overdose". Annals of Emergency Medicine. 9 (11): 588–90. doi:10.1016/s0196-0644(80)80232-0. PMID 7001962.
- ^ Paśko P, Rodacki T, Domagała-Rodacka R, Owczarek D (December 2016). "A short review of drug-food interactions of medicines treating overactive bladder syndrome". International Journal of Clinical Pharmacy. 38 (6): 1350–1356. doi:10.1007/s11096-016-0383-5. PMC 5124029. PMID 27738922.
- ^ Rosow CE (September 1997). "Anesthetic drug interaction: an overview". Journal of Clinical Anesthesia. 9 (6 Suppl): 27S–32S. doi:10.1016/S0952-8180(97)00124-4. PMID 9278852.
- ^ "IBM Watson Health Products". www.micromedexsolutions.com. Retrieved 2021-03-26.
- ^ Keisu M, Wiholm BE, Palmblad J (October 1990). "Trimethoprim-sulphamethoxazole-associated blood dyscrasias. Ten years' experience of the Swedish spontaneous reporting system". Journal of Internal Medicine. 228 (4): 353–60. doi:10.1111/j.1365-2796.1990.tb00245.x. PMID 2266345. S2CID 29753376.
